[NEWSMP] The maximum reimbursement amount for Chong Kun Dang's Lucentis biosimilar Lucen-BS will be reduced by 50%, while the maximum reimbursement amount for JW Pharmaceutical's Dulackhan Easy Syrup will be increased by 20.24%.
Novartis' Luxturna, a treatment for inherited retinal dystrophy, Bayer's Kerendia for diabetic nephropathy, Takeda's Obizur for hemophilia A, and Pfizer's Zavicefta for multidrug-resistant infections are newly listed on the pharmaceutical benefit list.
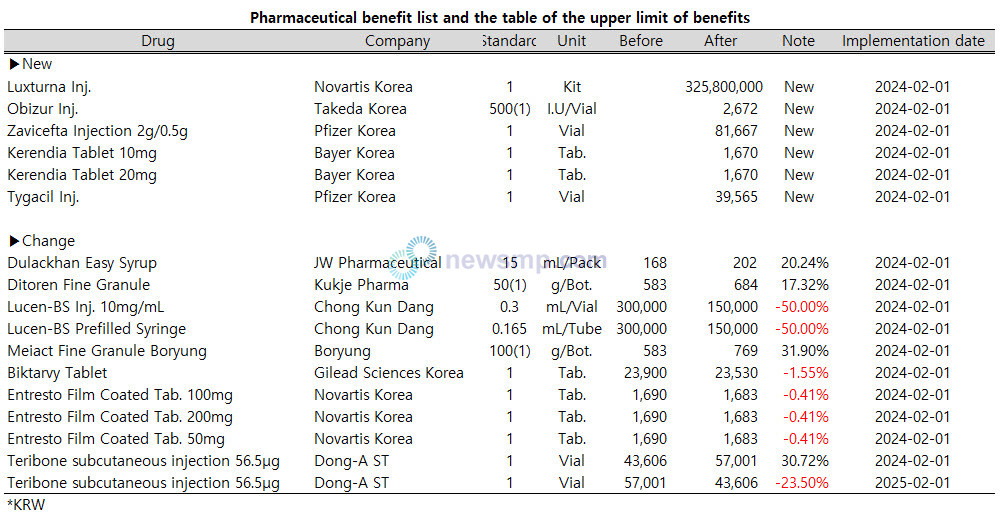
The Ministry of Health and Welfare announced a revision to the 'Pharmaceutical benefit list and the table of the upper limit of benefits' on January 26.
The maximum reimbursement amount for Luxturna is set at 325.8 million won per kit, Obizur at 2,672 won per 500IU (vial), Zavicefta 2g/0.5g at 81,667 won per vial, and Tygacil at 39,565 won per 50mg vial.
The cost of Kerendia is capped at KRW 1,670 per tablet, for both 10 mg and 20 mg.
Dulackhan Easy Syrup's maximum reimbursement cap will be raised from 168 won to 202 won per 15ml package, while the reimbursement ceilings for Ditoren Fine Granule (Kukje Pharma) and Meiact Fine Granule Boryung (Boryung) remain the same.
Teribone subcutaneous injection 56.5μg (Dong-A ST) will maintain its current reimbursement ceiling, but the price per vial will be decreased by 23.5% to 44,406 won on February 1 2025.
Effective next month, the reimbursement amount for Lucen-BS Inj. 10mg/mL and Lucen-BS Prefilled Syringe will be reduced by 50% from 300,000 won to KRW 150,000 won.
Moreover, the HIV treatment Biktarvy (Gilead Sciences) will see a 1.55% decrease in its reimbursement rate, changing from 23,900 won to 23,530 won, and three Entresto products, 50 milligrams, 100 milligrams, and 200 milligrams, will all be reduced from 1,690 won to 1,683 won, a decrease of 0.41% each.


