[NESMP] The number of clinical trial approvals granted by the Korean Ministry of Food and Drug Safety (MFDS) in the third quarter increased significantly from the same period last year but decreased significantly from the previous quarter.
According to the MFDS information platform (https://nedrug.mfds.go.kr/), a total of 124 institutions in South Korea received approval for 250 clinical trial plans from MFDS.
The number of approvals in South Korea in the third quarter of this year was up 40% from the same period last year, but down 40% from the previous quarter.
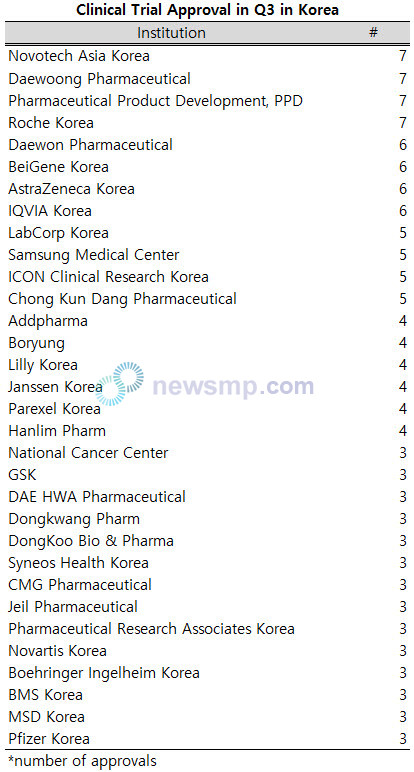
Novotech Asia Korea, Daewoong Pharmaceutical, PPD, and Roche Korea tied for the lead with 7 approvals each.
Daewoong Pharmaceutical, BeiGene Korea, AstraZeneca Korea, and IQVIA Korea each received six approvals, followed by LabCorp Korea, Samsung Medical Center, ICON Clinical Research Korea, and Chong Kun Dang Pharmaceutical with five approvals.
Moreover, Addpharma, Boryung, Lilly Korea, Janssen Korea, Parexel Korea, and Hanlim Pharm each secured four approvals.
Following the top performers, the National Cancer Center, GSK, DAE HWA Pharmaceutical, Dongkwang Pharm, DongKoo Bio & Pharma, Syneos Health Korea, CMG Pharmaceutical, Jeil Pharmaceutical, Pharmaceutical Research Associates Korea, Novartis Korea, Boehringer Ingelheim Korea, BMS Korea, MSD Korea, and Pfizer Korea each received three approvals.


