[Newsmp] Almost half of the medications approved by the Ministry of Food and Drug Safety (MFDS) in the first quarter are diabetes treatments, including dapagliflozin (brand name: Forxiga, AstraZeneca) and sitagliptin (brand name: Januvia, MSD).
According to the MFDS information platform (https://nedrug.mfds.go.kr/), a total of 471 medications, including narcotics, biologics, and advanced biopharmaceuticals, were granted approval during the first quarter.
By major ingredients, SGLT-2 inhibitor dapagliflozin recorded the highest number with a total of 124 items, including combination drugs, while sitagliptin followed closely with 113 items.
There were 35 products that were combinations of these two ingredients, and more than 200 products contained at least one of these two ingredients.
The ARB (angiotensin receptor blocker) hypertension medication, fimasartan (brand name: Kanarb, Boryung Pharmaceutical), also received approval for 66 products, and the CCB (calcium channel blocker) hypertension medication, amlodipine (brand name: Norvasc, Viatris), granted approval for 64 products.
In addition, despite the COVID-19 pandemic nearing its end, 27 new products containing acetaminophen (brand name: Tylenol, Janssen) have received new approval.
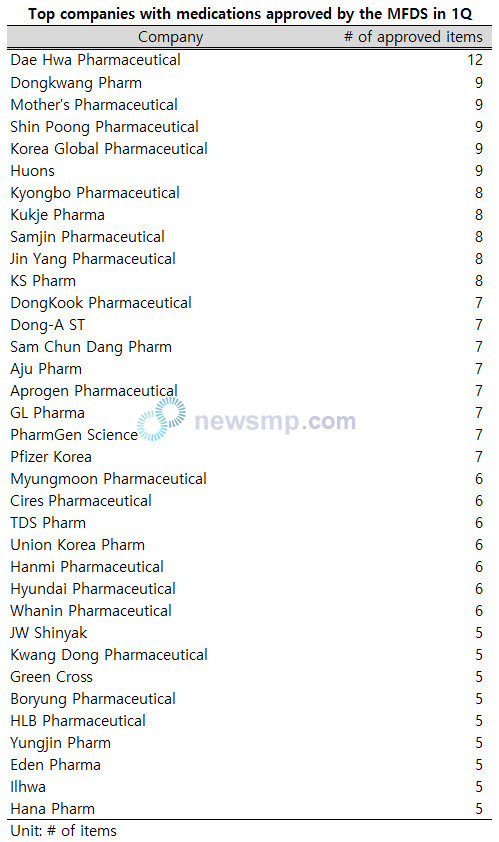
Among the total of 147 companies that received approval for at least one item, 35 companies received approval for five or more items.
Dae Hwa Pharmaceutical recorded the only two-digit figure with 12 approved products, while Dongkwang Pharm, Mother’s Pharmaceutical, Shin Poong Pharmaceutical, Korea Global Pharmaceutical, and Huons followed with a total of nine approved items.
Five companies including Kyongbo Pharmaceutical, Kukje Pharma, Samjin Pharmaceutical, Jin Yang Pharmaceutical, and KS Pharm obtained approvals for 8 items, while eight companies, such as DongKook Pharmaceutical, Dong-A ST, Sam Chun Dang Pharm, Aju Pharm, Aprogen Pharmaceutical, GL Pharma, PharmGen Science, and Pfizer Korea were approved for seven products.
Moreover, seven companies, including Myungmoon Pharmaceutical, Cires Pharmaceutical, TDS Pharm, Union Korea Pharm, Hanmi Pharmaceutical, Hyundai Pharmaceutical, and Whanin Pharmaceutical were granted approval for six products, and nine companies, including JW Shinyak, Kwang Dong Pharmaceutical, Green Cross, Boryung Pharmaceutical, HLB Pharmaceutical, Yungjin Pharm, Eden Pharma, Ilhwa, and Hana Pharm, received approval for five products.


