210 sitagliptin class including combination drugs… Combination of Zemiglo + Dapagliflozin appears
[Newsmp] In the first half, DPP-4 inhibitors and SGLT-2 inhibitors accounted for more than half of the drugs approved by the Ministry of Food and Drug Safety (MFDS).
A total of 879 drugs received MFDS approval in South Korea by the 22nd of this year, of which 449 drugs were DPP-4 inhibitors or SGLT-2 inhibitors, according to the MFDS information platform (https://nedrug.mfds.go.kr/).
The MFDS granted the permit to 83 companies for one or more DPP-4 inhibitors or SGLT-4 inhibitors, with an average of 5.37 drugs per company.
Ahn-Gook Pharmaceutical received 14 approvals for DPP-4 inhibitors or SGLT-2 inhibitors, Daehan New Pharm 13, Aju Pharm 11, MEDICA KOREA, PharmGen Science, Daewoong Bio, Sinil Pharmaceutical, and Jin Yang Pharmaceutical 10.
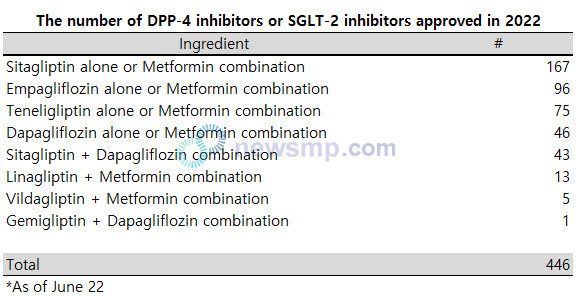
By ingredient, the DPP-4 inhibitor sitagliptin (Januvia, Organon) has been approved for 167 products including metformin combinations (excluding SGLT-2 combinations).
Including the SGLT-2 inhibitors dapagliflozin (Forxiga, AstraZeneca) combinations, 210 sitagliptin medications were approved in the first half.
Following sitagliptin, 96 medications were approved for empagliflozin (Jardiance, Boehringer Ingelheim), an SGLT-2 inhibitor.
For dapagliflozin, a total of 86 medications were granted, including 46 medications for a single drug or metformin combination and 43 medications for a sitagliptin combination.
The DPP-4 inhibitor Teneligliptin (Tenelia, Handok) followed with 75 medications.
Moreover, 13 medications of Linagliptin (Trajenta, Boehringer Ingelheim), and five medications of Vildagliptin (Galvus, Novartis) were approved, and recently, the domestic new drug Zemiglo (Ingredient, Gemigliptin) and dapagliflozin combination Zemidapa (LG Chem) also joined the competition.


