This year, more than 2,000 drugs have been approved by the Ministry of Food and Drug Safety (MFDS).
According to the MFDS’s information platform (https://nedrug.mfds.go.kr/), a total of 2029 drugs (including narcotics, advanced therapy medicinal products, biopharmaceutical drugs) from 256 companies were licensed by 24 December 2021.
A total of 1963 drugs, 34 biopharmaceutical drugs, 18 advanced therapy medicinal products, and 14 narcotics were counted.
By company, 85 companies received more than 10 approvals, and 19 companies had more than 20.
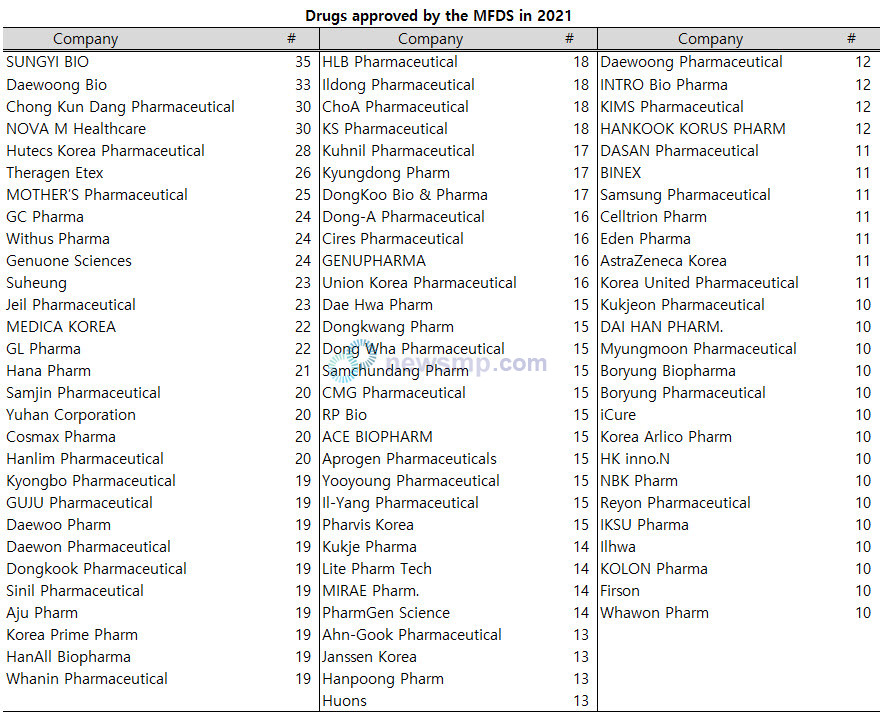
The company that received the most approvals was SUNGYI BIO with 35, followed by Daewoong Bio with 33, and Chong Kun Dang Pharmaceutical and NOVA M Healthcare with 30.
Hutecs Korea Pharmaceutical had 28 and ranked in the top 10 with Theragen Etex with 26, MOTHER’S Pharmaceutical with 25, and GC Pharma, Withus Pharma and Genuone Sciences with 24.
Suheung and Jeil Pharmaceutical followed with 23, MEDICA KOREA and GL Pharma with 22, Hana Pharm with 21, and Samjin Pharmaceutical, Yuhan Corporation, Cosmax Pharma and Hanlim Pharmaceutical with 20.
Moreover, 10 companies, including Kyongbo Pharmaceutical, GUJU Pharmaceutical, Daewoo Pharm, Daewon Pharmaceutical, Dongkook Pharmaceutical, Sinil Pharmaceutical, Aju Pharm, Korea Prime Pharm, HanAll Biopharma, and Whanin Pharmaceutical, were licensed 19 drugs.
There are 18 approvals for HLB Pharmaceutical, Ildong Pharmaceutical, ChoA Pharmaceutical, and KS Pharmaceutical, 17 for Kuhnil Pharmaceutical, Kyungdong Pharm and DongKoo Bio & Pharma, and 16 for Dong-A Pharmaceutical, Cires Pharmaceutical, GENUPHARMA, and Union Korea Pharmaceutical.
Dae Hwa Pharm, Dongkwang Pharm, Dong Wha Pharmaceutical, Samchundang Pharm, CMG Pharmaceutical, RP Bio, ACE BIOPHARM, Aprogen Pharmaceuticals, Yooyoung Pharmaceutical, Il-Yang Pharmaceutical, and Pharvis Korea were approved for more than 15 drugs.
Kukje Pharma, Lite Pharm Tech, MIRAE Pharm., and PharmGen Science had 14, Ahn-Gook Pharmaceutical, Janssen Korea, Hanpoong Pharm, and Huons 13, and Daewoong Pharmaceutical, INTRO Bio Pharma, KIMS Pharmaceutical, and HANKOOK KORUS PHARM 12.
In addition, DASAN Pharmaceutical, BINEX, Samsung Pharmaceutical, Celltrion Pharm, Eden Pharma, AstraZeneca Korea, and Korea United Pharmaceutical had 11 approvals.
Kukjeon Pharmaceutical, DAI HAN PHARM., Myungmoon Pharmaceutical, Boryung Biopharma, Boryung Pharmaceutical, iCure, Korea Arlico Pharm, HK inno.N, NBK Pharm, Reyon Pharmaceutical, IKSU Pharma, Ilhwa, KOLON Pharma, Firson, and Whawon Pharm were approved for 10 drugs.


