By ingredient, 31 Empagliflozinㆍ16 Amlodipine
In the third quarter, a total of 121 drug-related data were listed on the Korean version of Orange Book.
In 2019, the Ministry of Food and Drug Safety established the K-Orange Book with the aim of strengthening access to generic drug-related information.
K-Orange Book provides data such as ▲generics approved in Korea ▲compared with reference drugs ▲bioequivalence test methods and results (AUC, Cmax) ▲permission information (efficacy/effect, usage/dose, precautions for use).
Data on 121 drugs of a total of 53 companies and 29 ingredients have been added in the third quarter.
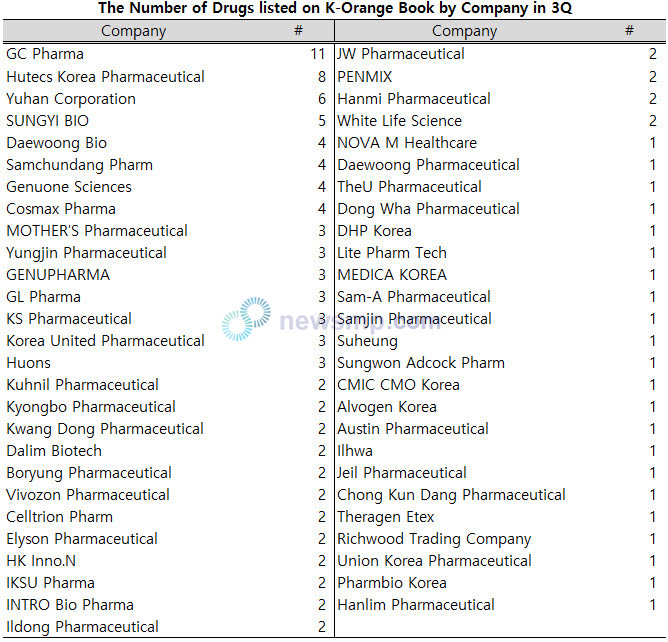
GC Pharma registered a total of 11 cases, the only one exceeding 10, followed by Hutecs Korea Pharmaceutical with 8, Yuhan Corporation with 6, and SUNGYI BIO with 5.
Daewoong Bio, Samchundang Pharm, Genuone Sciences, and Cosmax Pharma added 4 drugs, and MOTHER’S Pharmaceutical, Yungjin Pharmaceutical, GENUPHARMA, GL Pharma, KS Pharmaceutical, Korea United Pharmaceutical, and Huons listed 3 drugs.
Kuhnil Pharmaceutical, Kyongbo Pharmaceutical, Kwang Dong Pharmaceutical, Dalim Biotech, Boryung Pharmaceutical, Vivozon Pharmaceutical, Celltrion Pharm, Elyson Pharmaceutical, HK Inno.N, IKSU Pharma, INTRO Bio Pharma, and Ildong Pharmaceutical posted 2 drugs.
Moreover, the following companies added one drug on the K-Orange Book: Daewoong Pharmaceutical, TheU Pharmaceutical, Dong Wha Pharmaceutical, DHP Korea, Lite Pharm Tech, MEDICA KOREA, Sam-A Pharmaceutical, Samjin Pharmaceutical, Suheung, Sungwon Adcock Pharm, CMIC CMO Korea, Alvogen Korea, Austin Pharmaceutical, Ilhwa, Jeil Pharmaceutical, Chong Kun Dang Pharmaceutical, Theragen Etex, Richwood Trading Company, Union Korea Pharmaceutical, Pharmbio Korea, and Hanlim Pharmaceutical.

By Ingredient, Empagliflozin accounted for more than a quarter of the total number of registered cases with 31, while Amlodipine Besylate had 16.
Rivaroxaban was followed by 13 cases, Telmisartan 12 cases, Fluoxetine Hydrochloride 7 cases, and Topiramate 7 cases.
Tadalafil, Finasteride, and Pitavastatin Calcium were counted as 4 cases, Dobesilate Calcium Hydrate as 3 cases, Raloxifene Hydrochloride, Levofloxacin Hydrate, and Methimazole as two cases.
In addition, Nafamostat Mesilate, Donepezil Hydrochloride Monohydrate, Dutasteride, Diclofenac Sodium, Rebamipide, Levodropropizine, Rosuvastatin Calcium, Linagliptin, Metronidazole, Azelnidipine, Arformoterol tartrate, Afloqualone, Ezetimibe, Clopidogrel Bisulfate, Travoprost, and Paricalcitol were listed by one.


