15 Advanced biopharmaceuticalsㆍ9 Biologics
Jardiance generics (Ingredient: Empagliflozin, Boehringer Ingelheim) accounted for nearly 10% of drugs approved by the Ministry of Food and Drug Safety (MFDS) in the third quarter.
A total of 352 drugs (excluding herbal medicines) were approved, according to the MFDS.
Two-thirds (235) of them were prescription drugs, while non-prescription drugs (94) accounted for 26.7%, the remaining 23, or 6.5%, were material medicines.
Advanced biopharmaceuticals, including the Inherited retinal dystrophy (IRD) treatment, Luxturna (Ingredient: Voretigene neparvovec, Novartis), have been approved for 15 drugs, and 9 biologics such as Shingrix, a vaccine for the prevention of shingles (herpes zoster), were licensed.
By ingredient, Empagliflozin was the most common by 31, followed by hypertension and dyslipidemia triple combination therapies such as Amlodipine/Valsartan/Rosuvastatin, Amlodipine/Valsartan/Atorvastatin, and Telmisartan/Ezetimibe/Rosuvastatin by 12 drugs. In addition, 12 drugs of Fluoxetine have been approved.
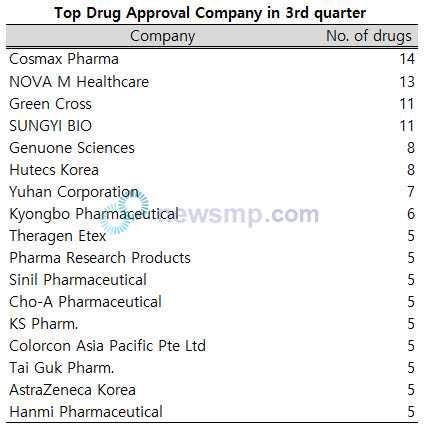
By company, Cosmax Pharma had the most by 14, NOVA M Healthcare by 13, and Green Cross and SUNGYI BIO by 11, with four companies licensed more than 10.
Genuone Sciences and Hutecs Korea had 8 drugs, Yuhan Corporation 7, Kyongbo Pharmaceutical 6, and Theragen Etex, Pharma Research Products, Sinil Pharmaceutical, Cho-A Pharmaceutical, KS Pharm., Colorcon Asia Pacific Pte Ltd, Tai Guk Pharm., AstraZeneca Korea, and Hanmi Pharmaceutical were 5 drugs.


